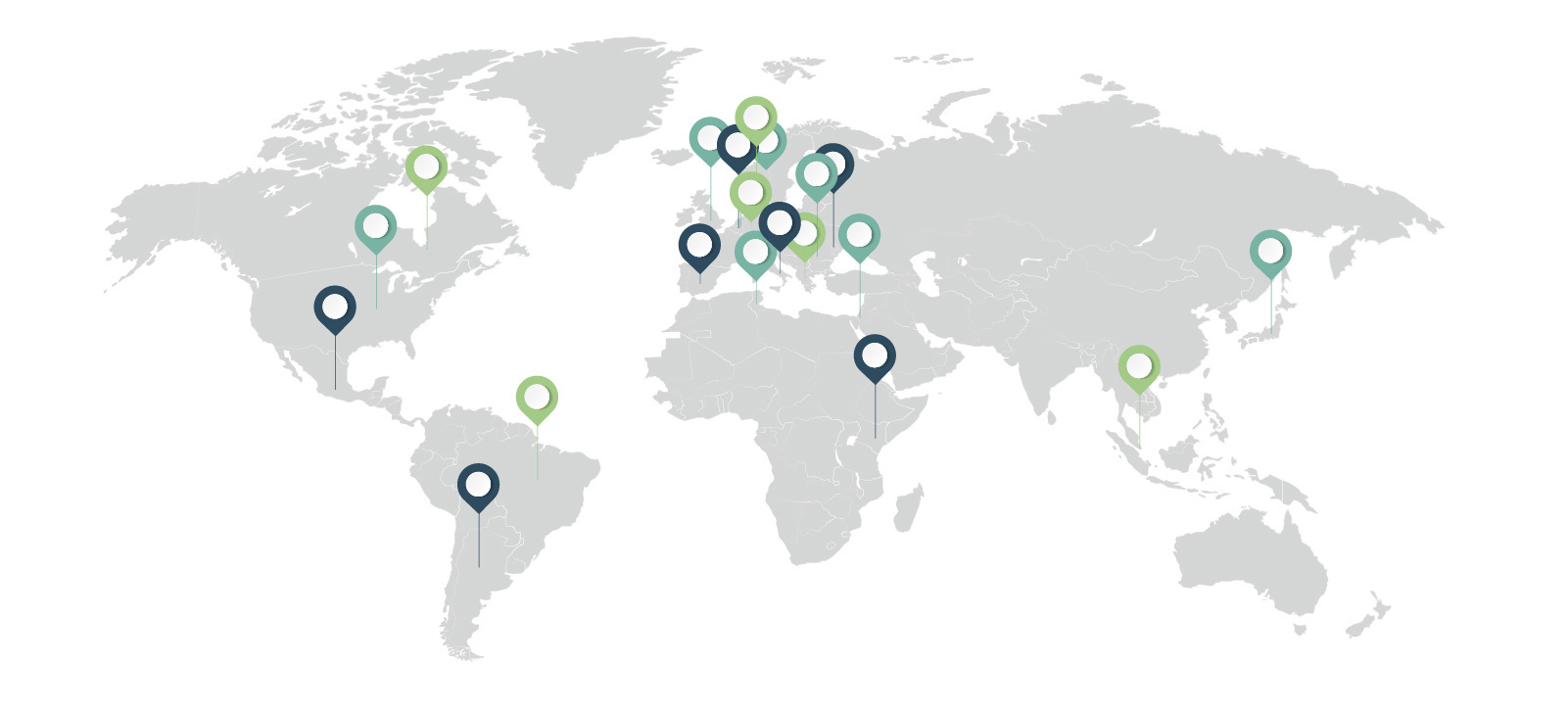
IDEOM Around the World
- Argentina
- Belgium
- Brazil
- Canada
- Denmark
- Germany
- Greece
- Israel
- Italy
- Japan
- Kenya
- Mexico
- Netherlands
- Romania
- Singapore
- Slovenia
- Spain
- Switzerland
- Tunisia
- United Kingdom
- United States
International Dermatology Outcome Measures
Bringing stakeholders together.
Patients
Industry
Patient Advocacy Groups
Healthcare Providers
Non Profits
Methodologists
Health Economists
Food & Drug Administration
Our Mission
IDEOM strives for the establishment of patient-centric outcomes to enhance the research and treatment of dermatological conditions.
News & Events



Everett C. Fox MD Memorial Award recipient Alice B. Gottlieb, MD, PhD, FAAD

Joseph F. Merola, M.D. M.M.Sc., appointed as the new Chair of the Department of Dermatology

April W. Armstrong, MD, MPH Appointed as Chief, Division of Dermatology


Dr. Alice B. Gottlieb Receives the #AAD2023 Master Dermatologist Award.
Congratulations, President Dr. Alice B. Gottlieb on receiving the #AAD2023 Master Dermatologist Award at the Stars of the Academy ceremony. This prestigious honor is in recognition of your commitment and service to #dermatology.
A Note From Our President
"The true finish line is when the patient gets to the right doctor and the right treatment and their disease has minimal to no impact on their quality of life."
- Alice Gottlieb, MD, PhD
Thank you to our Generous Sponsors
Platinum



Gold

Silver



 /
/

Bronze



Friend




Thank you to our supporters!


